OXIDATION OF 3,5,3′,5′-TETRA-TERT-BUTYL-4,4′-DIHYDROXYBIPHENYL WITH ATMOSPHERIC OXYGEN IN THE ABSENCE OF BASE CATALYST
S.V. Bukharov, L.K. Fazlieva, N.A. Mukmeneva, R.M. Akhmadullin, V.I. Morozov
Abstract
Use of dimethylformamide as solvent and preliminary addition of 3,5,3′,5′-tetra-tert-butyl-4,4′-diphenoquinone allow oxidation of 3,5,3′,5′-tetra-tert-butyl-4,4′-dihydroxybiphenyl with atmospheric oxygen to be efficiently performed in the absence of base catalyst.
The use of oxidants capable of regeneration by oxidation of their reduced forms with atmospheric oxygen shows promise in industrial oxidations. Such oxidants can be used repeatedly without accumulation of their reduced forms, which often form the main body of production waste.
One of such oxidants is a mild dehydrogenating agent, 3,5,3′,5′-tetra-tert-butyl-4,4′- diphenoquinone I [1]. Its reduced form, 3,5,3′,5′-tetra-tert-butyl-4,4′-dihydroxybiphenyl II, can be readily oxidized with atmospheric oxygen in the presence of base catalysts [2] [scheme (1)].
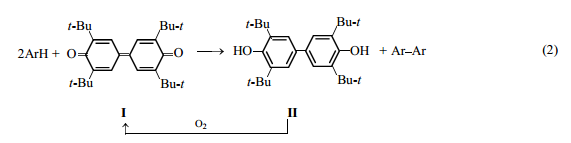
The use of alkalis as ionizing agents catalyzing oxidation of phenols has a significant drawback: The spent alkalis should be neutralized, with the formation of waste. At the same time, dipolar aprotic solvents [3] forming hydrogen bonds with sterically hindered phenols [4] facilitate to some extent their ionization.
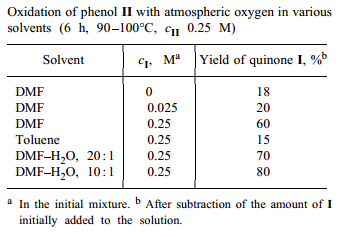
We found that, with dimethylformamide or its mixture with water as solvent, oxidation of II with atmospheric oxygen can be performed at relatively low temperatures in the absence of base catalysts. Since it is known that quinones can both catalyze [5,6] and inhibit [7] autooxidation of sterically hindered phenols, we also examined the effect of quinone I on oxidation of II. We found that addition of I in amounts comparable with those of II appreciably accelerates oxidation of II (see table)
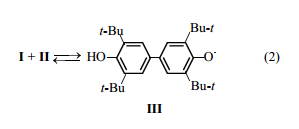
Redox reactions between sterically hindered phenols and quinones with the formation of phenoxyl radicals were reported in [8-11]. Such a transformation in the system phenol II-quinone I was discussed in [12], and formation of phenoxyl radical III [scheme (2)] was presumed.
This equilibrium can account for the effect of I on oxidation of II. Namely, in this first stage, radicals III are generated by relatively fast dehydrogenation of the substrate, whereas in the absence of I radicals III are generated by slow [13] oxidation of un0ionized phenol II with atmospheric oxygen [scheme (3)]. This is follower by reactions (4) and (5).
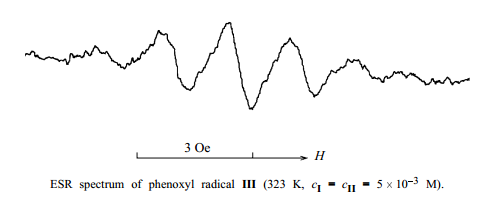
The fact that, for significant acceleration of the oxidation of II, the amount of I should be comparable with that of II, and not catalytic, counts in favor of such a mechanism. Furthermore, we detected radicals III in toluene solutions containing equimolar amounts of II and I by ESR spectroscopy (see figure). The multiplicity of the signal (1:4:6:4:1) and its parameters (hyperfine coupling constant 1.67 Oe, g-factor 2.004) coincide with the published data [14]. The low intensity of the signal, decreasing with temperature, shows that the concentration of radicals III is low and is determined by the equilibrium constant of reaction (2).
Oxidation of II in the presence of I can be regarded as a self-conjugated process in which oxidation of II with atmospheric oxygen is conjugated with its dehydrogenation under the action of I.
Thus, regeneration of quinone I from its reduced form II can be efficiently performed with atmospheric oxygen in DMF in the absence of base catalysts. Preliminary addition of quinone I results in noticeable acceleration of the reaction owing to the occurrence of a self-conjugated process.
Experimental
The ESR spectra of III were recorded on a Radiopan SE/X-2544 spectrometer. The solution was deaerated by threefold freezing-pumping-thawing.
Oxidation of 3,5,3′,5′-tetra-tert-butyl-4,4′-dihydroxybiphenyl II. A solution of 1 g of phenol II and 1 g of quinone I in 10 ml of dimethylformamide was placed in a reactor equipped with an efficient reflux condenser and an adapter for air bubbling. Through the reaction mixture heated to 90-100°C, air was bubbled for 6 h at a rate of 39 1h-1. Then the mixture was cooled to room temperature, and the precipitate of I was filtered off.
Oxidation of II in other solvents and without addition of I was performed similarly.
REFERENCES
1. Mukmemeva, N.A., Bukharov, S.V., Kadyrova, V.Kh., Zharkova, V.M., Gorshunova, N.R.-S., and Fazlieva, L.K., Zh. Obshch. Khim., 1996, vol. 66, no. 9, pp. 1526-1529.
2. US Patent 4482754, 1984, Ref. Zh. Khim., 1985, 21N172P.
3. Bukharov, S.V., Konoshenko, L.V., Solov’eva, S.E., Gainullin, V.I., Syakayev, V.V., Mannafov, T.G., Chugunov, Yu.V., and Samuilov, Ya.D., Zh. Obshch. Khim., 1999, vol. 69, no. 1, pp. 130-133.
4. Borisover, M.D., Stolov, A.A., Kudryavtsev, V.Yu., and Solomonov, B.N., Zh. Fiz. Khim., 1991, vol. 65, no. 2, pp. 312-315.
5. Gersmann, H.R. and Bickel, A.F., J. Chem. Soc., 1959, no. 9, pp. 2711-2716.
6. Conrady, J.J. and McLaren, G.A., J.Am. Chem. Soc., 1960, vol. 82, no. 17, p. 4745.
7. Strigun, L.M., Vartanyan, L.S., and Emanuel’, N.M., Usp. Khim., 1968, vol. 37, no. 6, pp. 969-997.
8. Mazaletskaya, L.I., Karpukhina, G.V., and Lazarev, G.G., Izv. Akad. Nauk SSSR, Ser. Khim., 1989, no. 5, pp. 1188-1190.
9. Denisov, E.T., Kinet. Katal., 1997, vol. 38, no. 6, pp. 832-838.
10. Prokof’ev, A.I., Malysheva, N.A., and Bubnov, N.N., Izv. Akad. Nauk SSSR, Ser. Khim., 1976, no. 3, pp. 511-515.
11. Prokof’ev, A.I., Usp. Khim., 1999, vol. 68, no. 9, pp. 806-816.
12. Shanina, E.L., Zaikov, G.E., Mukmeneva, N.A., Polym. Degrad. Stab., 1996, vol. 51, no. 1, pp. 51-56.
13. Ershov, V.V., Nikiforov, G.a., and Volod’kin, A.a., Prostranstvenno-zatrudnennye fenoly (Sterically Hindered Phenols), Moscow: Khimiya, 1972.
14. Pokhodenko, V.D., Degtyrev, L.S., Koshechko, V.G., and Kuts, V.S., Problemy khimii svobodnykh radikalov (Problems of Free Radical Chemistry), Kiev: Naukova Dumka, 1984.