IMPROVED METHOD FOR SEPARATE DETERMINATION OF SULFUR COMPOUNDS IN HYDROCARBON GASES
A. G. Akhmadullina, G. M. Nurgalieva, L. N. Orlova and I. K. Khrushcheva
UDC 543.257.1:543.272.5
Sulfur compounds in hydrocarbon gases produced in petroleum refining consist mainly of hydrogen sulfide and mercaptans, with small amounts of carbonyl sulfide and carbon disulfide. The separate determination of these compounds, when all are present, is a difficult analytical problem. Of the methods used to analyze for these compounds [1-3], the potentiometric method [1] is of the greatest interest from the standpoint of availability of reagents and apparatus. However, it has a number of disadvantages, such as the relatively poor accuracy in the determination of mercaptan sulfur concentration when the hydrogen sulfide concentration in the gas is 4-10 times that of the mercaptan sulfur, the use of toxic methanol and a concentrated ammonia solution in the potentiometric analysis of the absorber solution of carbonyl sulfide, and the unsuitability of the method for the analysis of gases containing carbon disulfide along with the carbonyl sulfide. The carbon disulfide is also absorbed by the ethanol solution of monoethanolamine (MEA), and it can also interact with the titrant; consequently, unduly high results are obtained in the potentiometric determination of carbonyl sulfide.
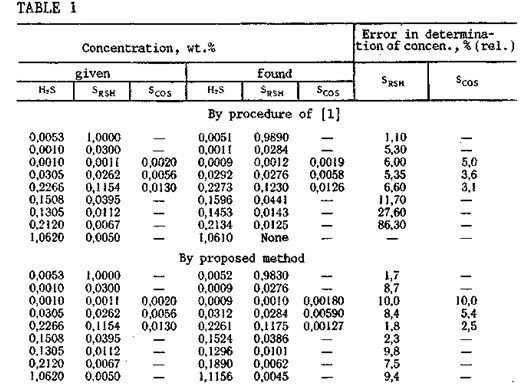
In order to eliminate the influence of hydrogen sulfide on the determination of mercaptan sulfur content, we have proposed that the single absorber solution (40% NaOH) should be replaced by two different solutions used in series: a 3% aqueous Na2C03 solution for se¬lective removal of the hydrogen sulfide, and a 40% aqueous NaOH solution for absorption of the mercaptans and residual hydrosulfide. The use of these two solutions can give a significant improvement in the accuracy of determination of the mercaptan sulfur concentration in gases, and it also offers a means for expanding the limits of applicability of the method in the analysis of gases with various ratios of hydrogen sulfide and mercaptan sulfur concentrations (Table 1). It has also been established that carbonyl sulfide is not absorbed by these solutions.
In order to eliminate the use of a concentrated ammonia solution in each potentiometric determination, it is proposed to use as the titrant an aqueous solution of ammoniacal silver nitrate (GOST 22985-78) in place of the aqueous or alcoholic solution of silver nitrate.
As the background solution in the potentiometric titration of carbonyl sulfide, the toxic ammonia-methanol solution is replaced by a 5% solution of MEA in ethanol. In the titration of carbonyl sulfide on this background, the initial potential was established within the same limits (from -430 to -300 mV) as when the ammonia-methanol background solution is used. At the equivalent point in the titration of the carbonyl sulfide, the potential jump in the region from -350 to -30 mV reaches an absolute magnitude of 200-300 mV.
For the determination of carbonyl sulfide concentration in gases in the presence of carbon disulfide, a spectrophotometric method is proposed in place of the potentiometric method. The use of an ethanol solution of MEA as the absorber solution for carbonyl sulfide and carbon disulfide, in place of the ethanol solution of diethylamine (DEA) that was used in [2], gives a much faster determination of carbonyl sulfide and carbon disulfide in gases. The rate of passage of the gas through the DEA solution is no greater than 5 liters/h [2], whereas the rate through the MEA solution may be 100 liters/h or even higher. The absorptivities of carbonyl sulfide and carbon disulfide in alcoholic solutions of MEA and DEA are practically identical (Table 2).

The light absorption curves for carbonyl sulfide and carbon disulfide in a 5% MEA solution have very distinct maxima at respective wavelengths of 230 and 290 nm (Fig. 1).
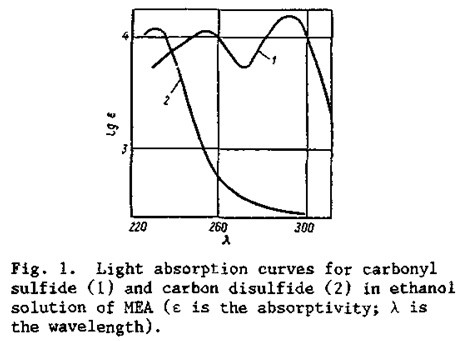
The alcoholic MEA solution is optically transparent in this region, and the dependence of its absorbance on the concentrations of carbonyl sulfide and carbon disulfide follows the Lambert-Beer law.
With carbonyl sulfide and carbon disulfide concentrations of 0.047% and 0.025% by weight, respectively, in a model 5% alcoholic MEA solution, the corresponding concentrations deter¬mined spectrophotometrically were 0.051% and 0.022% (mean results of three determinations). The corresponding relative errors of determination are 7.84% for the carbonyl sulfide and 12% for the carbon disulfide.
Thus, the improved method for the separate determination of hydrogen sulfide, merceptans, and carbonyl sulfide in gases consists of passing the gas, taken in a type PU-50, PU-400, or PGO-50 sampler (GOST [All-Union State Standard] 14921-78) through a series of three absorber bottles, the first with a 3% sodium carbonate solution, the second with a 40% aqueous NaOH solution, and the third with a 5% alcoholic MEA solution; the gas is passed through at a rate of 100-200 liters/h, after which the absorber solutions are titrated with aqueous ammoniacal silver nitrate in a pH-340 apparatus with silver sulfide and silver chloride electrodes. In the analysis of LPG that does not contain any C5 or higher hydrocarbons, samples of the gas may be taken directly from the process line through a fitting in the top of a horizontal section of the pipe, while measuring the volume of sample passed through the apparatus by means of a gas meter.
In the analysis of gases with hydrogen sulfide concentrations that are smaller than, or commensurate with, the mercaptan sulfur concentration, a single absorber solution can be used for the two components — 40% NaOH.
In titrating hydrogen sulfide, the potential jump is observed in the region from -680/ -500 mV to -350/-150 mV; in the titration of mercaptan sulfur and carbonyl sulfide, it is observed in the region from -350/-200 mV to -50/+50 mV. In the determination of hydrogen sulfide and carbonyl sulfide, the sulfur equivalent is equal to 16; in the determination of mercaptan sulfur, it is equal to 32.
When carbon disulfide is present in the gas, the alcoholic MEA solution is analyzed in an SF-26 spectrophotometer on the basis of the absorbance at wavelengths of 230 and 290 nm. The concentration of carbonyl sulfide (Ccos) and the concentration of carbon disulfide (CCS2) in the solution is calculated by solving the system of equations
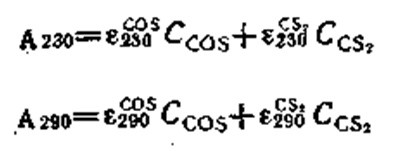
where A230 and A290 are the absorbances of the alcoholic MEA solution saturated with carbonyl sulfide and carbon disulfide, at respective wavelengths of 230 and 290 nm; eZ30» ?29o> e23o» and eff3 are the mean absorptivities of COS and CS2 in the alcoholic MEA solution at respective wavelengths of 230 and 290 nm.
LITERATURE
1. DIN [West German Standard] 51855, Bestimmung des Gehaltes an Schefeluerbindungen.
2. V. K. Novikov, L. A. Yarygina, Yu. M. Afanas’ev, et al., Zavod. Lab., No. 7, 792-793 (1972).
3. F. N. Pekhota, A. M. Kamenev, and G. M. Malyatova, Pererab. Gaza Gazov. Kondensata, No. A, 3-9 (1977).