DETERMINATION OF THIOSULFATE SULFUR IN OXIDIZED SOUR CAUSTIC WASTES
A. G. Akhmadullina, L. N. Orlova and G. A. Ostroumova
UDC 543.257.1:628.543
In sour caustic waste streams from refineries, the most harmful component is inorganic sulfides. The content of inorganic sulfides may be tens of thousands of times greater than the allowable limits [1], For the treatment and disposal of industrial waste streams, extensive use is made of simple and effective oxidation methods that will give partial or complete breakdown of toxic sulfur-containing compounds or will convert them to a nontoxic form. In the process of oxidative treatment, sulfides are converted to thiosulfates which do not have any odor and can be oxidized to harmless sulfates in biological treatment.
Of presently available methods for the quantitative determination of thiosulfates, some are complex and require the use of toxic reagents, the others are nonselective and require preremoval or masking of a number of interfering anions (iodometric methods [2] are an example of the latter type). Potentiometric methods are based on the use of toxic mercury salts as solution titrants [3, 4] and require premasking of the sulfite ion by formalin. Indirect methods of analysis for thiosulfate content [5, 6] ultimately come down to iodometry and are not free of its disadvantages.
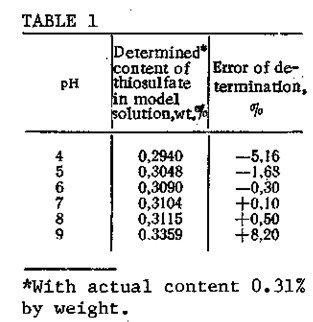
We have developed a method for the quantitative determination of thiosulfate sulfur in aqueous caustic solutions by titration with a 0.1 N AgN03 solution at pH 5-8 in a pH-340 potentiometer with silver chloride and silver sulfide electrodes. In the titration, a rela¬tively insoluble precipitate of the sulfur salt with the anion is formed. The completeness of silver thiosulfate precipitation depends on the pH of the medium (Table 1). At pH 5-8, maximum precipitation of the silver thiosulfate is achieved, and precipitation of silver hydroxide is completely avoided. Deviations of the pH from this range lower the accuracy of determination. Thus, the thiosulfate content should be determined in a neutral or slightly acidic medium. In an alkaline medium, silver nitrate hydrolyzes to precipitate silver hydroxide, so that the results obtained in the analyses will be higher than the true values. In order to avoid this, the titration with AgN03 solution in an alkaline medium is carried out with ammonia present in the titrant composition or in the support electrolyte for the titration. However, in determining the content of thiosulfate sulfur, the presence of ammonia cannot be tolerated, since it dissolves the silver thiosulfate precipitate that is formed in the titration and hence makes it impossible to perform the analysis.
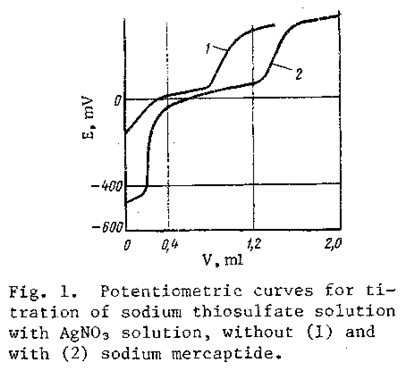
The required pH level is achieved by neutralizing the alkaline medium with acetic acid; the pH is determined by means of indicator paper or a pH meter. This creates a buffer medium in the solution, preventing any significant changes in pH during the course of the analysis. The analysis requires 10-15 min. The end of the precipitation of silver thiosulfate is accompanied by a sharp change in the potential of the solution in the region of 50-250 mV (Fig. 1, curve 1). The presence of sulfite and sulfate ions in the solution, or the presence of sodium mercaptide and sulfide, does not interfere with the determination if these substances are present in respective mole ratios no greater than 1:5 and 1:50 relative to the thiosulfate.
In the presence of sodium sulfide and mercaptide, the analysis is performed in two ways. On the first sample, the content of sulfide and mercaptide sulfur is determined by potentio-metric titration in accordance with GOST 22985—78. The thiosulfate. content is determined on the second sample in the following manner0 A 30-40 ml sample of 1 N KOH solution is placed in a titration beaker, its pH is adjusted to 5-7 by adding acetic acid, a sample of the solu¬tion to be analyzed is added, and the contents of the beaker are titrated with 0.1 N AgN03 solution on silver chloride and sulfide electrodes. The initial potential of the test solu¬tion stabilizes in the region from —700 to —740 mV when inorganic sulfides are present, and near —450 mV when mercaptides are present. The first jump of potential in the region from —650 to —680 mV and the second somewhat above —450 mV reflect the presence of residual quantities of sulfide and mercaptide in the neutral medium.
The content of thiosulfate sulfur (S, in %) is determined from the jump in potential in the 50-200-mV region on the basis of the formula:
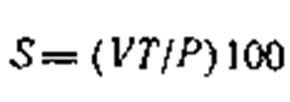
where V is the volume of AgN03 solution used in titrating the thiosulfate, ml; T is the titer of the AgN03 solution, referred to thiosulfate or mercaptan sulfur g/ml; P is the weight of the sample of substance analyzed, g.
The volume V, when sulfide or mercaptide is present in the test sample, is determined as the difference between the volumes of titrant consumed in titrating all of the sulfur-containing compounds and the sodium sulfide and/or mercaptide from the second sample (Fig, 1, curve 2).
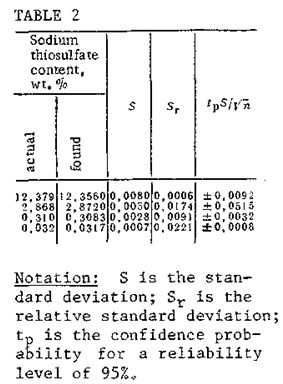
If there is no sodium or mercaptide in the mixture being analyzed, the analysis is performed in a single step by the method we have described. In this case, the initial potential of the solution levels out between -100 and -200 mV. In Table 2 we have listed data from a statistical workup of results obtained in determinations of sodium thiosulfate content by potentiometric titration with a number of determinations n=5 and pH 7. It can be seen that the standard deviations of the results of the determinations, within the recommended interval of concentrations, is no greater than 0„008. The mathematical treatment of the results of the determinations was performed in accordance with the recommendations of IUPAC [7].
The proposed method is suitable for the determination of the group composition of sulfur-containing compounds in industrial wastewater, for the analysis of oxidized sour caustic wastes to be directed to biological treatment, and for kinetic studies of oxidation reactions of inorganic sulfur compounds. In the use of this method to determine the content of thio-sulfate sulfur in sour caustic wastes from the Tuapse Petroleum Refinery after oxidative treatment in the laboratory, the standard deviation was no greater than 6%. The content of thiosulfate sulfur as determined by potentiometric titration was 3.75% by weight, and that determined iodometrically was 4.12% by weight.
LITERATURE
1. M. G. Rudin, G. A. Arsen’ev, and A„ V„ Vasil’ev, General Plant Facilities in Refineries [in Russian], Khimiya, Leningrad (1978)
2. Yu. Yu. Lur’e and A„ I. Rybnikova, Chemical Analysis of Industrial Wastewater [in Rus¬sian], Khimiya, Moscow (1974)
3. Referativ. Zh. Khim., 7178 (1977)
4. J. Havas, Hung. Sci„ Instrunu, No„ 5, 35-37 (1973)
5. USSR Inventor’s Certificate 548,808
6. W. P. Kilroy, Talanta, 25, No., 6, 359-362 (1978)
7. R. V. Fennel and T. S„ West, Zh. Anal. Khim., 2b_, No,, 5, 1021-1023 (1971)