CATALYTIC ACTIVITY OF MANGANESE AND COPPER OXIDES IN THE OXIDATION OF SULFUR COMPOUNDS
R. M. Akhmadullin, Dinh Nhi Bui, A. G. Akhmadullina, and Ya. D. Samuilov
Kazan National Research Technological University
Abstract
Catalytic activity of manganese and copper oxides in the oxidation of sulfur compounds, including sodium sulfide, sodium hydrosulfide and ammonium sulfide was studied. Polymeric catalyst based on copper(II) and manganese(IV) oxides showed high activity, especially in the oxidation of sodium hydrosulfide and ammonium sulfide. Kinetic investigation showed that all the reactions are first order with respect to oxygen concentration and zero—to the source concentration of sulfur compounds.
Cleaning sulfuralkaline waste containing toxic sulfide and hydrosulfide compounds is one of the major problems in refineries and petrochemical plants. Some methods for cleaning sulfuralkaline wastes, such as stripping, degassing, carbonization [1] require a large expenditure of energy and have environmental problems, because they cause secondary pollution of air with hydrogen sulfide and sulfur dioxide.
More promising is the method of catalytic oxidation of toxic sulfides by atmospheric oxygen to less harmful oxygencontaining sulfur compounds such as thiosul fate and sulfate.
In recent years, more widespread heterogeneous catalytic systems were formed by physical or chemical fixing catalyst complexes on the solid support of the polymer carrier. They allow avoiding a number of technical difficulties, including equipment corrosion and environmental pollution by heavy metal salts. These catalysts have high catalytic activity in a wide range of concentrations of oxidized compounds and pH, mechanical strength, chemical and hydrolytic stability, and can be used for 3–5 years. Polymeric catalysts can work in an alkaline medium at temperatures up to 100°С and pressures up to 7.0 kgf/cm2 and in intensive bubbling. Partial mechanical wear of catalyst granules do not reduce the catalytic activity, so the surface granule is regenerated and the new active centers are involved in the catalyst surface [2–4].
Previously [5], we studied kinetics of the oxidation of the sodium hydrosulfide in the presence of the heterogeneous catalyst based on copper and manganese oxides CuO-15/MnO2-5, which showed the highest activity. In view of the availability and the high activity of this catalyst, it have appropriated to investigate efficiency in the oxidation of other sulfide compounds — sodium sulfide and ammonium sulfide, which are most often found in waste water of oil refineries.
EXPERIMENTAL
The catalyst was prepared by introducing metal oxides into the polymer matrix in according to the method [6]. The metal oxide content, expressed as wt % of metal oxide in the catalyst samples, was determined by means of chemical analysis (example CuO-15/MnO2-5— the catalyst contains 15.0% cuprum oxide and 5.0% manganese oxide by mass).
All reagents were obtained from KAZPELEN and were of the highest grade available and used without further purification. During experiments, the following oxides of the metals with variable valence were used: MnO2 (Russia, GOST 4470–79), CuO (Russia, GOST 16539–79). The used polymer was a type of high density polymer made from polyethylene, known commercially as KAZPELEN no. 15313–003 (Russia, GOST 16337–77).
The sodium hydrosulfide and ammonium sulfide were prepared by dissolving in water, and the sodium sulfide was prepared by dilution of the 9-watered sodium sulfide in water under GOST 2053–77.
The oxidation of sulfur compounds was hold in an air bubbling vessel of oxidation tower in the presence of 5.0 g heterogeneous catalyst. The oxygen was fed into the reaction solution with a speed of 12.0 to 84.0 liter per hour. The solution inside the reactor was stirred at a speed of 1400 revolutions per minute. The temperature of the reaction solution was maintained at 60°С with thermally controlled magnetic mixer. Initial concentrations of sulfur compounds ranged from 0.4 to 1.5 wt %. Their concentration in the solution was determined by potentiometric titration according to GOST 22985–90.
RESULTS AND DISCUSSION
The Efficiency of a Heterogeneous Catalyst Based on Copper and Manganese Oxides in the Oxidation of Sulfide and Hydrosulfide Compounds
The oxidation process proceeds from sulfide (S2–) and hydrosulfide (HS–) to thiosulfate (S2O32-), sulfite (SO32-) and sulfate (SO42-) is expressed by the following equations:
2S2–(SH–) + 2O2 + H2O = S2O32-+ 2OH–, (I)
S2O32-+ 2OH– + 2O2 = 2SO42- + H2O, (II)
2S2–(SH–) + 3O2 = 2SO32- (III)
2SO32- + O2 = 2SO42- (IV)
S2–(SH–) + 2O2 = SO42-(V)
It is known that the reaction of Equation (IV) may proceed far more rapidly than that of Equations (II) and (III). As a result, a considerable amount of S2O32- is left in the solution together with SO42-, which is the final oxidized product.
It is known [7] that hydrosulfide and sodium sulfide are oxidized slowly in the absence of catalysts. Ammonium sulfide is not very stable, rapidly is oxidized even under normal conditions.
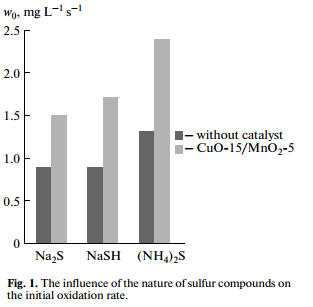
The investigation results of the oxidation of sodium sulfide, hydrosulfide and ammonium sulfide in the presence of heterogeneous oxide catalyst CuO-15/MnO2-5 and without catalyst are shown in Fig. 1. Initial concentrations of sulfur compounds were 0.4 wt %. The investigation results show that the catalyst accelerates the oxidation rate of sulfide, sodium hydrosulfide and ammonium sulfide. Thus, the rate of oxidation of sodium hydrosulfide and ammonium sulfide in the presence of the catalyst is 1.85 times higher than without catalyst, and catalytic oxidation rate is 1.66 times higher than the rate of noncatalytic oxidation in the case of sodium sulfide.
These results demonstrate a promise to apply the proposed catalyst in the detoxification of sulfidecon taining aqueous technological condensate and sulfur alkaline waste.
The Kinetics of the Oxidation of Sodium Sulfide, Sodium Hydrosulfide and Ammonium Sulfide in the Presence of Heterogeneous Catalysts Based on Oxides of Manganese and Copper
In homogeneous processes, the catalyst particles have molecular dimensions, and its activity is directly proportional to the active mass. In heterogeneous catalysis involves only the catalyst surface, which is not related to its total mass.
With intensive stirring, the oxidation of sulfur compounds in the presence of heterogeneous catalysts proceeds in the kinetic region. In this case, the concentration of reactants on the catalyst surface and in the solution is equal, but the reaction is only on the external surface. Then the process is determined by the kinetics of chemical reactions.
It is possible that not all atoms on the surface are active. Only some of them, which are called active sites, have the ability to form the active intermediates. The number of active sites may be depending on the method of preparation of the catalyst and concentration of a mixture of oxides of manganese and copper in the polymer matrix. In according to Taylor’s theory, the active sites of heterogeneous catalysts based on oxides of manganese and copper are the surface atoms of the crystal lattice. In these crystalline “peaks” there are free valence ions of manganese and copper, so they can form a reactive intermediate. Surface coordinatively–unsaturated transition metal ions in the intermediate oxidation state react with the reagent molecules to form active surface intermediates, which speeds up the catalytic reaction.
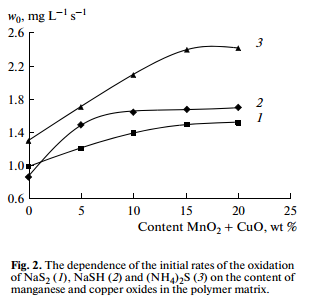
To select the optimal composition of a heterogeneous catalyst, we investigated the influence of the concentration of a mixture of oxides of manganese and copper in the polymer matrix (with a ratio of MnO2:CuO = 1: 3) on the reaction rate. Figure 2 shows that the rate of oxidation increases with increasing the concentration of the catalyst component in the polymer matrix from 0 to 15 wt %. A further increase in the concentration does not influence on the rate of oxidation, which is probably due to the saturation of the geometric surface of the catalyst active sites.
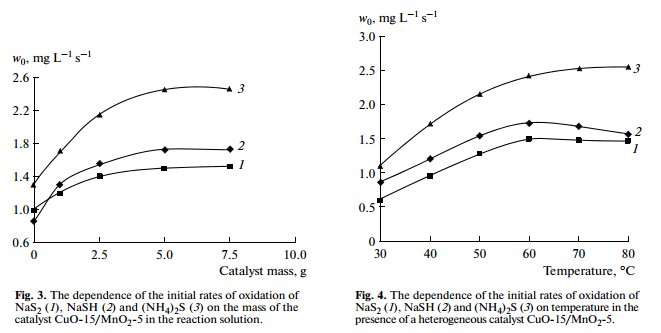
Amounts of heterogeneous catalyst CuO-15/MnO2-5 contained in the reaction solution have effect on the rate of oxidation of sulfide and hydrosulfide compounds. Increasing catalyst amount to 5.0 g leads to increase the rate of sodium sulfide oxidation. The further increases in catalyst amount don’t influence rate of reaction (Fig. 3), which, apparently, is explained by limiting hydrodynamics in the reactor.
In the absence of a catalyst, increasing temperature and pressure tends to increase the rate and depth of the oxidation of sulfide and hydrosulfide, but without changing the mechanism of the reaction [8]. In the presence of a catalyst CuO-15/MnO2-5 at atmospheric pressure, the rate of oxidation of sulfide and hydrosulfide increases with increase in temperature up to 60°С. A further increase in temperature do not influence on the rate of reaction. However, the rate of the oxidation of ammonium sulfide increases with increase in temperature above 60°С (Fig. 4). Apparently, this is explained by the fact that ammonium sulfide was decomposed into free hydrogen sulfide and ammonia, which are taken out from the reactor with excess oxygen. Experiments have shown that the loss of ammonium sulfide in solution is 2–3% at 60°С, and 8–10%—at 80°С.
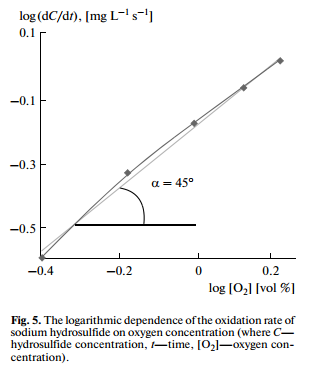
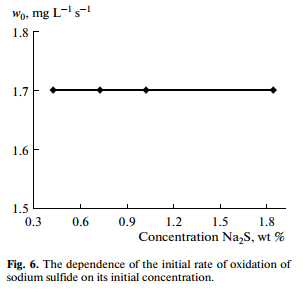
The reaction orders of the oxidation of sulfur compounds were determined by a differential method. The oxidation of sodium hydrosulfide had first order with respect to oxygen concentration (Fig. 5). The oxidation rate of sodium hydrosulfide, sodium sulfide and ammonium sulfide did not depend on their initial concentrations (Fig. 6), which indicated the zero order reactions with respect to sulfur concentration.
The previous results indicate that the oxidation of sulfide and hydrosulfide compounds is limited by the solubility of oxygen in water, the number of active sites on the catalyst surface and the surface area of the catalyst.
The data presented above suggest that the oxidation of sulfur compounds proceeds by the following mechanism:
Mn4+ + SH– = Mn3+ + SH0, (VI)
2SH0= HSSH, (VII)
Cu2+ + Mn3+ = Cu+ + Mn4+, (VIII)
4Cu+ + O2 = 4Cu2+ + 2O2–. (IX)
The first two stages (VI and VII) occur at a high rate, which is consistent with the zeroorder reaction with respect to the initial concentration of sulfur compounds. In stage (VIII), as proved by the authors [9, 10], there is a transfer of electrons between the copper and manganese cations in a mixture of singlephase oxides MnO2 and CuO. The ratelimiting step is (IX), in which there are the activation of oxygen and the oxidation of the copper ion.
So we have established that the polymeric catalyst based on copper and manganese oxides has highly activeness in the oxidation of sulfur compounds, including sodium sulfide and sodium hydrosulfide and ammonium sulfide. The highest rate is observed in the oxidation of sodium hydrosulfide and ammonium sulfide.
The optimal concentration of the catalytically active component in the polymer matrix and the optimal catalyst amount for maximum rate in the oxidation of sulfur compounds were experimentally found. It is found that all the reactions are of first order with respect to oxygen and zero—to the initial concentration of sulfur compounds.
REFERENCES
1. Linevich, S.N., Kompleksnaya pererabotka i ratsional’noe ispol’zovanie serovodosoderzhashchikh stochnykh vod (Integrated Processing and Rational Use of Wastewater), Moscow: Stroiizdat, 1987.
2. Yuffa, A.Ya., Geterogennye metallokompleksnye katalizatory (Heterogeneous Metal Complex Catalysts), Moscow: Khimiya, 1981.
3. Pomogailo, A.D., Polimernye immobilizovannye metallokompleksnye katalizatory (PolymerAnchored Metal Complex Catalysts), Moscow: Nauka, 1988.
4. Akhmadullina, A.G., Akhmadullin, R.M., Agadzhanyan, S.I., and Mukmeneva, N.A., Vestn. Tekhnol. Univ. (Kazan), 2009, vol. 1, no. 2, p. 64.
5. Akhmadullin, R.M., Bui Din’ N’i, Akhmadullina, A.G., Samuilov, Ya.D., in Vestn. Tekhnol. Univ (Kazan), 2012, vol. 15, no. 1, p. 50.
6. USSR Inventor’s Certificate no. 1041142, Byull. Izobret., 1983, no. 34.
7. Zamyshlyaeva, I.M., Ochistka proizvodstvennykh stochnykh vod (Treatment of Industrial Wastewater), Moscow: Stroiizdat, 1969.
8. Ksandopulo, S.Yu., Shurai, S.P., and Barko, A.B., Vestn. Kuban. Gos. Tekhnol. Univ., 2003, p. 35.
9. Veprek, S., Cocke, D.L., Kehl, S., and Oswald, H.R., J. Catal., 1986, vol. 100, p. 250.
10. Buciuman, F.C., Patcas, F., and Hahn, T., Chem. Eng. Process., 1999, vol. 38, p. 563.